
Nearly half of all breast imaging facilities undergoing their annual MQSA inspections were found to have at least one or more deficiencies related to the Enhancing Quality Using the Inspection Program (EQUIP)in the first six months after its launch. According to the FDA, out of the 4,166 annual MQSA inspections performed, deficiencies related to EQUIP were found in 44% of those inspections.
Three new inspection questions
For those not familiar with EQUIP, the program consists of an additional 3 questions (and subquestions) to the annual MQSA inspection to insure facilities are adhering to the highest quality standards when assessing the quality of their mammographic images.
In effect since January 1, 2017, the goal of EQUIP is to prepare facilities to address image quality on a continual basis and include the lead interpreting physician and/or interpreting physician as the person ultimately responsible for the image quality.
The good news for facilities is that 2017 is considered an implementation year, giving facilities time to address deficiency findings before January 1, 2018, when inspection citations will be issued.
The added questions and subquestions to the annual MQSA inspection pertaining to EQUIP are as follows:
1. Does the facility have procedures for corrective action (CA) when clinical images are of poor quality?
(a) Do the procedures include a mechanism for providing ongoing IP feedback on image quality to RT’s or other designated facility personnel?
(b) Do the procedures require documenting any corrective actions taken and documenting the effectiveness of any corrective actions taken?
2. Does the facility have procedures to ensure that clinical images continue to comply with the clinical image quality standards established by the facility’s accreditation body?
(a) Do the procedures include a mechanism for regular reviews of image quality attributes of a sample of mammograms performed by each active RT and a sample of mammograms accepted for interpretation by each active IP?
(b) Is there documentation of such review since the last inspection?
3. Does the facility have a procedure for LIP oversight of QA/QC records and corrective actions?
(a) Does the procedure include requirements for LIP oversight of QA/QC records, including review of the frequency of performance of all required tests?
(b) Does the procedure include requirements for LIP review to determine whether appropriate corrective actions were performed when needed?
Where deficiencies have been found in first 6 months
The biggest percentage of deficiencies with EQUIP involve processes and documentation as evidenced by the graph below.
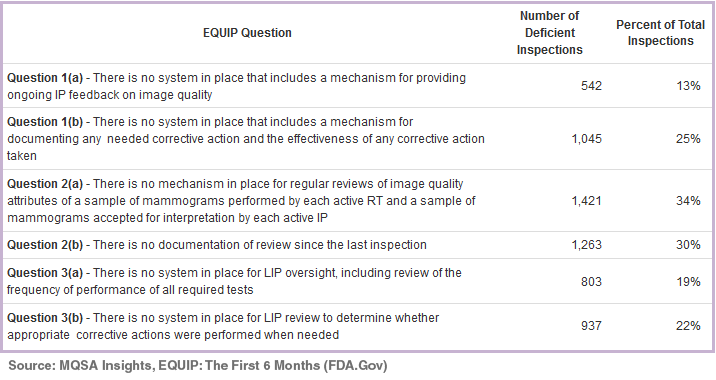
Confusion regarding quality assessments
Image quality, according to the FDA, is a shared responsibility between interpreting physicians and mammography technologists. The EQUIP questions are to determine that each facility is taking the time through a formal process to assess and document the overall image quality for the facility as a whole.
Taking a look at the MQSA's FAQ page on EQUIP, the confusion may lie in the fact that the guidelines are fairly ambiguous and subject to interpretation. Facilities are free to design processes that best fit their individual situations and processes.
For example, you are not required to have a written procedure - you may verbally communicate your system for providing ongoing feedback, documenting, and assessing corrective actions.
The FDA states that it is only looking to ensure processes are in place for image quality review, not to review specific details. But they are looking for written documentation of periodic clinical image quality reviews as evidenced by question 2b. I think you can safely assume that if it wasn't documented, it didn't happen in the eyes of the inspector. The FDA then goes on to explain that if your reviews are performed in huddles or meetings, a simple summary form, statement written by the LIP, meeting records, and even memos to staff are considered sufficient documentation.
It's up to you to determine what your procedure is, how you will document it, and then ensure that all in your department know and adhere to that procedure.
Where you can find additional guidance on EQUIP
I found the FAQ page a great resource in nailing down specifics of what is expected and who needs to do what and when. It's a great place to start.
 The FDA encourages breast imaging facilities to utilize your inspector as a resource during this educational year.
The FDA encourages breast imaging facilities to utilize your inspector as a resource during this educational year.
In addition, they have established an MQSA Facility Hotline for both phone (1-800-838-7715) and email (MQSAhotline@versatechinc.com). They also have a variety of educational resources posted on the MQSA website to help you understand this initiative better.
Several MQSA consultants and mammography educators such as BIS- Breast Imaging Specialists, AHEC (Advanced Health Education Center), and Mammography Educators have added EQUIP to their training services.
Our best advice is to speak with your peers at other facilities who have already undergone their inspection to learn what went well for them and what did not.
After all, we're all in this together when it comes to better image quality and the early detection of breast cancer.
Related articles:

Mary Lang Pelton
Director of Marketing Communications
